Académie d'Excellence "Complexité et diversité du vivant"
8th Academy 4 Research Seminar - Ion Channel - Oct 18th, 2022
- Research
- Education
- Science and society
- IDEX
- International
on the October 18, 2022
Campus Valrose, Online
Visioconference Zoom (registration for private codes on demand)
It is our pleasure to announce the Academy 4 Research Webinars on Bio-Medical & Transdisciplinary Topics.
They take place on the 3rd Tuesday of the Month.
The 8th session on "ION CHANNEL" took place on the 18th of October 2022 at noon (12pm) at Salle des Actes du Grand Château, VALROSE Campus and remotely via Zoom.
Thank you to Professor VIANA and the participants despite the national strike, the shortage of gasoline, the COVID19, etc...



Here is the link to the video recording of Professor VIANA's excellent lecture and the following questions / discussion.

Pr Felix VIANA de la IGLESIA, visiting scientist to Dr Guillaume SANDOZ' lab (iBV)
From : Instituto de Neurociencias, Universidad Miguel Hernández - CSIC, San Juan de ALICANTE, SPAIN.
Email : felix.viana@umh.es
Team website : http://in.umh.es/
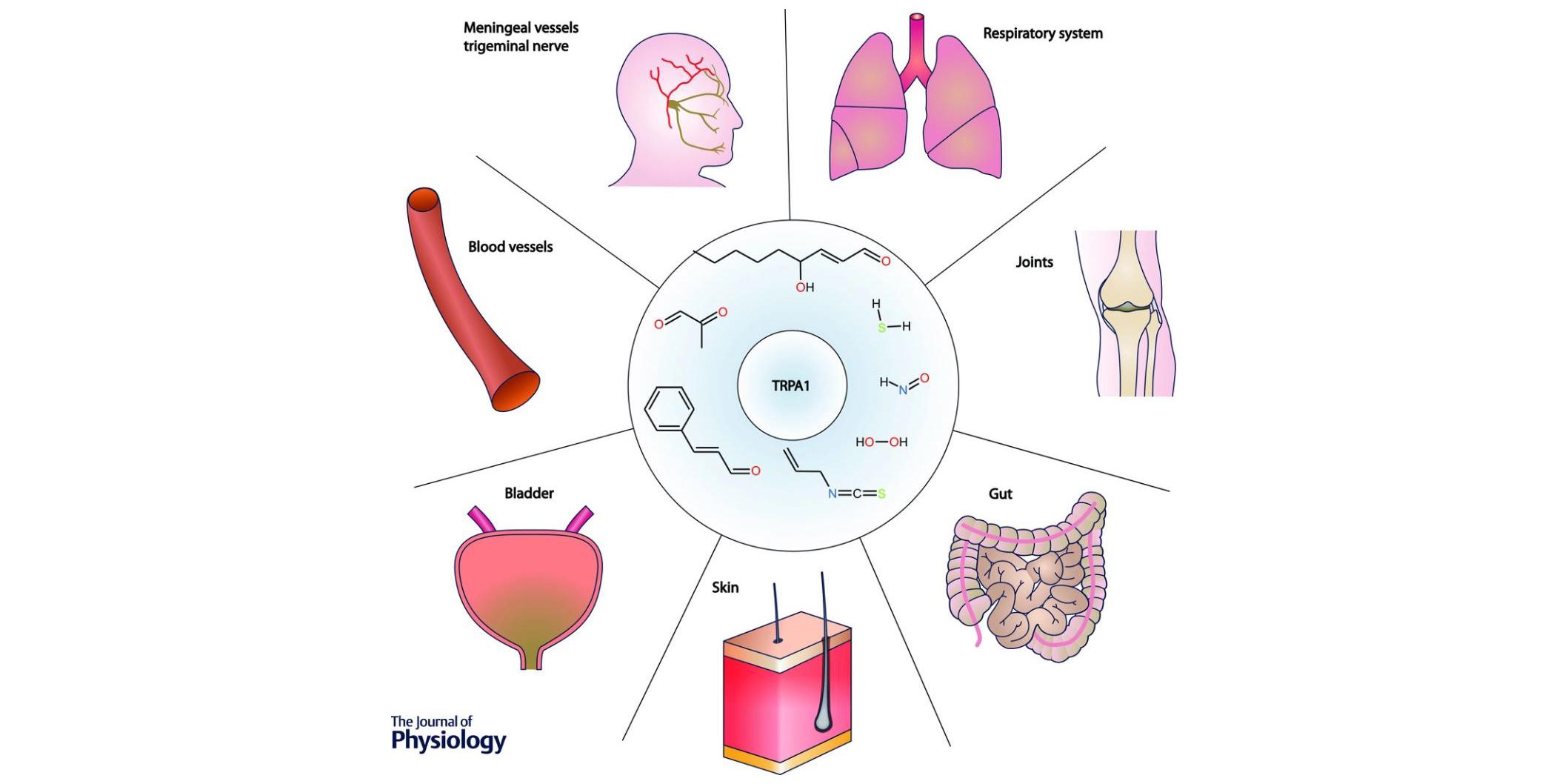
Title of the lecture: "Pathophysiology of TRPA1 Channels: Role in Neuropathic and Inflammatory Pain"
Abstract: The transient receptor potential Ankyrin 1 (TRPA1) channel, is a polymodal non-selective cation channel expressed in many barrier tissues, including cutaneous nociceptive nerve endings. Originally described as a sensor for noxious cold (DOI: 10.1016/s0092-8674(03)00158-2), it is now recognized as a broad-band transducer of physical and irritant chemical signals, acting as a molecular sentinel of cellular stress and tissue damage (DOI: 10.1113/JP270935). In this talk, I will review some the work performed in our laboratory over the past 15 years, trying to understand its multiple functions. This journey will stop at different locations: its role as cold sensor in viscera, its activation by harmful products, including bacterial endotoxins and its promiscuous activation by clinically approved drugs. The end of the trip will highlight our recent discovery of the molecular interaction of TRPA1 with the sigma1 receptor, a chaperone broadly expressed in many human tissues, and the potential to harness the downmodulation of TRPA1 offered by this interaction as a preventive strategy for chemotherapy-induced painful peripheral neuropathy (https://doi.org/10.1093/brain/awac273).
Bio: I have a long standing interest in the structure and function of ion channels, molecular machines that regulate the flow of ions across cell membranes. After medical studies (U. Santiago) and a PhD in Physiology and Biophysics (U. of Washington), I was a postoc with Bertil Hille (U. of Washington) and Bernd Nilius (KULeuven). I joined the IN in 1998 and established my research group in 2001. We try to understand how neurons sense external stimuli, focusing on the role played by ion channels in thermosensation and pain. We pioneered the characterization of mammalian cold thermoreceptors (Nat Neurosci 2002, J Neurosci 2006) and made important contributions to understanding the role of shaker-like potassium channels in their excitability. We found that a tight balance between expression of TRPM8, a cold-activated TRP channel and Kv channels fine tunes their thermal sensitivity, expanding the operating range of a single thermotransducer from the innocuous to the noxious range (J Neurosci 2009). In other studies, we combined electrophysiological recordings and computational techniques to define the role of HCN channels in the oscillatory behavior and coding of cold temperature signals by trigeminal neurons (J Physiol 2009, J Neurophysiol 2012). Together with my colleague Carlos Belmonte, we have studied the role of TRPM8 channels in the firing of sensory nerve endings in the cornea and were able to establish their critical role in tearing (Nat Med 2010) and eye blinking (Nat Commun 2015). More recently, we used fluorescent cell sorting techniques combined with array profiling to define the channelome of mouse cold thermoreceptors, uncovering the role of TASK-3, a pH-sensitive two-pore domain potassium channel, in their excitability (Cell Rep 2014). During the past decade we also made significant contributions to our current understanding of the molecular structure of TRPM8 (J Biol Chem 2014) and its post-translational modifications (J Biol Chem 2009, 2012).
Another focus of our work has been the characterization of TRPA1, a nociceptive TRP channel activated by natural and industrial electrophilic agents. Early work from our group established the key role of this channel in visceral cold sensing (J Neurosci 2008). In addition, we demonstrated the participation of TRPA1 in unwanted side effects of clinically relevant drugs, including the 1, 4 dihydropyridines (Channels 2007), the antiepileptic phenytoin (J. Dental Rsearch, 2017) and the anticanter agent oxaliplatin (manuscript in preparation). More recently, we discovered that TRPA1 is the target of endotoxins produced by Gram-bacteria. Activation of TRPA1 is partially responsible for the symptoms that accompany bacterial infections: pain, neurogenic inflammation, neuropeptide release and peripheral vasodilation. These findings (Nat Commun 2014) are relevant for understanding the pathological mechanism of septic shock and its treatment and has led to the filing of a patent.
Along the way, we paid attention to other aspects of ion channel regulation. Together with A. Gomis and D. Bayliss, we found a direct modulation of TASK-3 channels by Gq proteins (Proc Natl Acad Sci USA 2006). We also discovered that TRPM8 channels are important in the growth and proliferation of prostate cells (Pflügers Arch 2011, PLoS One 2012).
Currently, together with the laboratory of Ana Gomis, we are using genetic labeling strategies to identify subpopulations of primary sensory neurons involved in the sensory manifestations of chronic pain. To this end, we are applying a combination of techniques: behavioral testing, calcium imaging, biochemistry and molecular profiling (RNASeq) to different animal models of chronic pain (diabetic neuropathy, chemotherapy-related pain, arthritic pain), trying to establish the role of specific ion channels and receptors in the different phenotypes characteristic to each pathology (e.g. mechanical, thermal hypersensitivity). Another recent focus of our group is the use of novel optogenetic and imaging techniques to interrogate neural circuits participating in various sensory behaviors and homeostatic loops. Using appropriate Cre-driver lines we achieved the selective expression of ChR2, Halorhodopsin, GCamp6, etc, in defined cell populations in the peripheral and central nervous system and are trying to establish their contribution to chronic pain, itch, thermoregulation and fluid balance.
At the institutional level, I made significant efforts to promote education and research in the field of ion channels. I co-organized the first meeting of the Spanish Network of Ion Channels (RECI) that met in Alicante in 2007. Together with Dr. Antonio Ferrer Montiel I started the Workshop on Ion Channels and Transporters that biennially brings together researchers from Spain, Italy, Portugal and France. With Dr. David Julius (Shaw Prize in Life Science and Medicine and Prince of Asturias Award for Technical and Scientific Research) I also co-organized two major international meetings on TRP channels. Since 2015 I am a member of the Scientific Advisory Board of the French LabEx “Ion Channel Science and Therapeutics” scientific consortium. I have served on several national and international (EU; France) funding committees and expert panels (ANR France, Welcome Trust UK, etc)
Further relevant Bibiography:
- Viana, F. and Hille, B. (1996) Modulation of high-voltage-activated calcium channels by somatostatin in acutely isolated rat amygdaloid neurons. J. Neuroscience 16:6000-6011.
- Viana, F., De la Peña, and Belmonte, C. (2002) Specificity of cold thermotransduction is determined by differential ionic channel expression. Nature Neuroscience 5:254-260.
- Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. (2006) Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. Journal of Neuroscience 26:12512-12525.
- Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, Viana F, Garrison JC, Bayliss DA. Inhibition of a background potassium channel by Gq protein alpha-subunits (2006). Proc Natl Acad Sci U S A. 103, 3422-3427.
- Fajardo O, Meseguer V, Belmonte C, Viana F. (2008) TRPA1 channels mediate noxious cold sensing in mammalian vagal sensory neurons. Journal of Neuroscience 28:7863-7875.
- Madrid R, de la Peña E, Donovan-Rodriguez T, Belmonte C, Viana F. (2009) Variable threshold of cold-sensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. Journal of Neuroscience 29:3120-3131.
- Talavera K, Karashima Y, Vanoirbeek JAJ, Damann N, Meseguer V, Everaerts W, Benoir M, Vennekens R, Viana F, Nemery B, Voets T, Nilius B. (2009) Nicotine activates the chemosensory cation channel TRPA1. Nature Neuroscience 12:1293-1299.
- Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C. (2010) Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nature Medicine 16:1396-1399.
- Meseguer V, Alpiza YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernández-Peña C, Talavera A, Kichko T, Navia B, Sánchez A, Señarís R, Reeh P, Pérez-García MT, López-López JR, Voets T, Belmonte C, Talavera K, Viana F. (2014) TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nature Communications DOI: 10.1038/ncomms4125.
- Morenilla-Palao C, Luis E, Fernandez-Peña C, Quintero E, Weaver JL, Bayliss DA, Viana F (2014). Ion channel profile of TRPM8 cold receptors reveals a role of TASK-3 potassium channels in thermosensation. Cell Reports 8(5):1571-82. doi: 10.1016/j.celrep.2014.08.003.
- Quallo T, Vastani N, Horridge E, Gentry C, Parra A, Moss S, Viana F, Belmonte C, Andersson DA, Bevan S (2015). TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nature Communications 6:7150. doi: 10.1038/ncomms8150.
- Viana F. TRPA1 channels: molecular sentinels of cellular stress and tissue damage (2016). Journal of Physiology 594, 4151-4169. doi: 10.1113/JP270935.
- Lolignier S; Gkika D; Andersson D, Enrico Leipold E, Vetter I, Viana F, Noël J, Busserolles J. New Insight in Cold Pain: Role of Ion Channels, Modulation, and Clinical Perspectives (2016). Journal of Neuroscience (2016), 36, 11435-11439.
- Reimúndez A, Fernández-Peña C, García G, Fernández R, Ordás P, Gallego R, Pardo-Vazquez JL, Arce V, Viana F, Señarís R (2018). Deletion of the cold thermoreceptor TRPM8 increases heat loss and food intake leading to reduced body temperature and obesity in mice. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.3002-17.2018
Please feel free to contact felix.viana@umh.es or guillaume.sandoz@univ-cotedazur.fr to make appointment for further discussion with Prof VIANA.
--------------------
ORGANIZERS:
Academy of Excellence 4 "Complexity & Diversity of the Living Systems"
Institut de Biologie de VALROSE iBV
PARTNERS
Academy of Excellence 5 "Human Societies, Ideas and Environments"
Graduate School and Research HEALTHY - Health Science Ecosystems
Graduate School and Research LIFE - Life and Health Sciences
Institute NeuroMod - Cognitive Systems, Normality and Pathology of the Human Brain and Computational Neurosciences
Labex SIGNALIFE - Network for Innovation on Signal Transduction Pathways in Life Sciences