Académie d'Excellence "Complexité et diversité du vivant"
9th Academy 4 Research Seminar on "Membrane Biology & Imaging" - Melody SUBRA (SOPHIA) / Dr Daniel LEVY (PARIS) - 21/02/2023 - Théâtre du grand Château VALROSE NICE
- Research
- Education
- Science and society
- IDEX
- International
on the February 21, 2023
Campus Valrose
It is our pleasure to announce the next Academy 4 Research Webinars on Bio-Medical & Transdisciplinary Topics.
The transdisciplinary Conference on "Membrane Biology & Imaging" will take place on the 21st of February 2023 at noon (12pm) at VALROSE Campus, théâtre du grand Château.
Attendance upon free registration here / Entrée gratuite sur inscription.
For more details, see below or here : https://univ-cotedazur.fr/recherche-innovation/structures-de-recherche/academies-dexcellence/academie-4/melody-subra-daniel-levy-membrane-biology-imaging-9th-academy-4-research-seminar-february-21st-2023-theatre-valrose
PROGRAMME:
12:00

Melody SUBRA, IDEX & FRM PhD student at Université Côte d'Azur, Teacher assistant at Université Côte d'Azur
PhD supervisor : Dr Bruno MESMIN, CR CNRS IPMC
From : Molecular and Cellular Pharmacology Institute (IPMC) CNRS, laboratory of Dr Bruno ANTONNY, "Dynamics of lipids membranes and protein coats", 660 route des lucioles SOPHIA-ANTIPOLIS
Email : subra@ipmc.cnrs.fr
Team website : Team of Dr Bruno Antonny "Dynamics of lipid membranes and protein coats"
Title of the lecture:
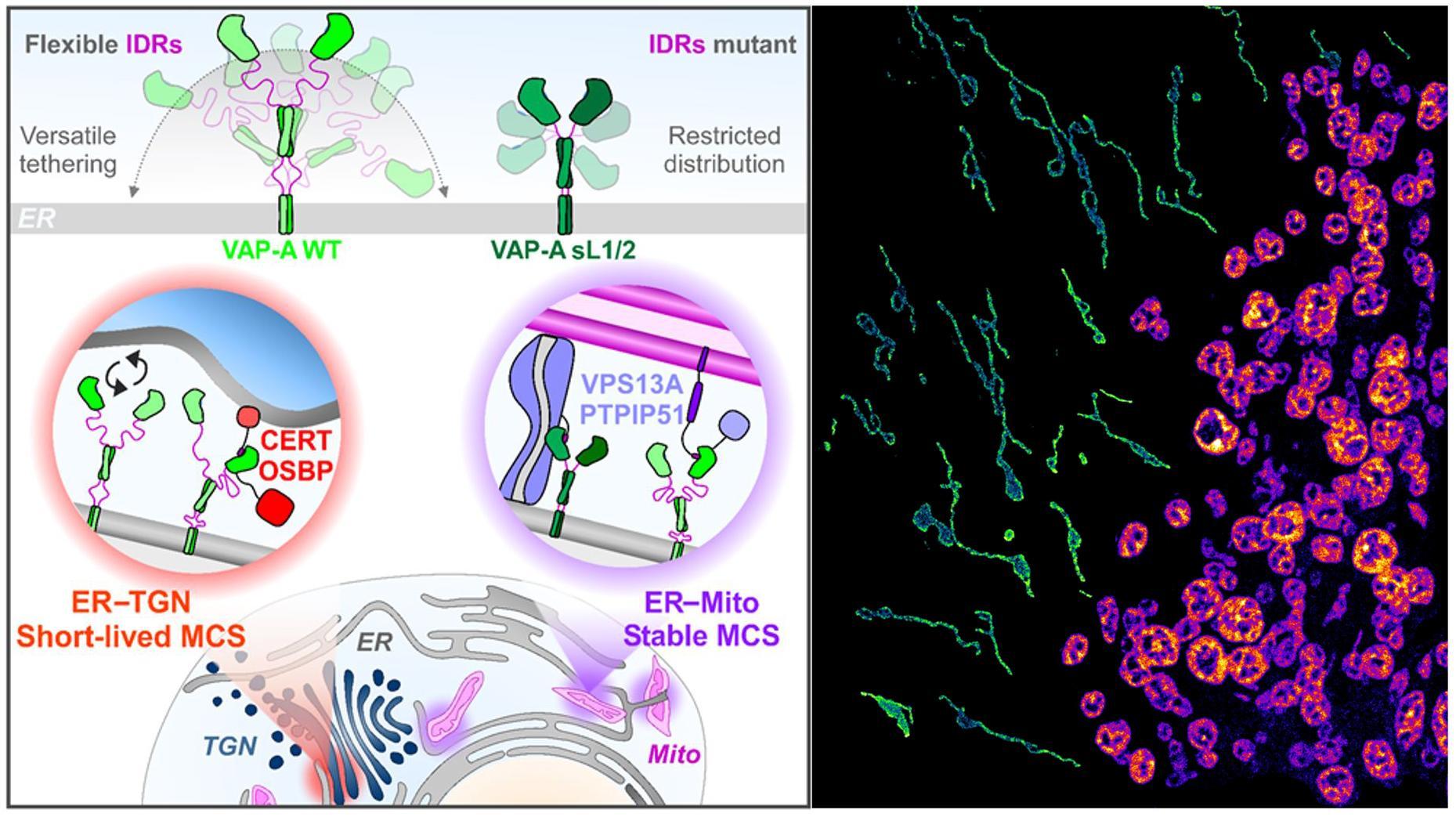
"VAP-A intrinsically disordered regions enable versatile tethering at membrane contact sites"
Abstract:
VAP-A is a receptor at the surface of the endoplasmic reticulum (ER) for hundreds of proteins containing a FFAT motif and having a wide range of structures and functions. VAP-A is also required for creating multiple membrane contact sites (MCS) between the ER and other compartments, which notably enable non-vesicular lipid exchanges between membranes. For example, the lipid-transfer protein (LTP) OSBP interacts with VAP at ER/Golgi MCS to transport cholesterol through coupled counter-exchange and hydrolysis of PI(4)P. It is well known that VAP-A partners contain a FFAT motif specifically recognized by the Major-Sperm-Protein (MSP) domain of VAP, however, how this receptor adapts to its different targets in MCSs that are so different in geometry and lifetime is not understood. In this study, we show that VAP-A contains two intrinsically disordered linkers that provide it with a high degree of flexibility to enable functional organization of different MCSs. A VAP-A mutant without flexible linkers is restricted in its subcellular localization and does not support lipid transport by OSBP and CERT at ER/Golgi MCS. However, this mutant is present at ER/mitochondria MCS by interacting with VPS13A and PTPIP51, and thus facilitates lipid transport contributing to cardiolipin metabolism and mitochondrial fusion. In conclusion, this work indicates that VAP-A conformational flexibility mediated by its intrinsically disordered regions is key to ensure membrane tethering especially at short-lived MCSs; it also demonstrates the implication of VAP-A in mitochondrial fusion.
Biography:
Melody SUBRA is a teaching assistant and a PhD student at Université Côte d'Azur supported by IDEX & FRM fundings.
She received her High School Diploma in health and social sciences, with honors at Simone Weil High school, Dijon, France in 2014 and the 1st prize in the general high school competition, health and social sciences at la Sorbonne, Paris. She then received in 2017 her Bachelor’s Degree (French Licence) in Biochemistry and Molecular Biology, with honors at Bourgogne university, Dijon and her Master's Degree in Biology Health & Environment, Signaling and Integrated Systems in Biology, option cellular determinism, with honors at Ecole pratique des hautes études, Paris.
Melody SUBRA did her Master I Internship at IPMC, where she studied the mechanism of action of anticancer agents targeting the OSBP protein in Dr Antonny's Lab in 2018. She then did her Master II Internship at C3M Mediterranean Center for Molecular Medicine, Nice, on the development of mutated melanocyte cell lines by Crispr/Cas9 in S. Rocchi & T. Passeron Lab in 2019.
In 2019, she received her PhD fellowship from Academy of Excellence 4 of IDEX Université Côte d'Azur to work on membrane biology under the supervision of Drs Bruno ANTONNY & Bruno MESMIN at IPMC, Sophia ANTIPOLIS.
Since then, she won in December 2021, the Jury Prize: MICA image competition, curiosities of research of Université Côte d'Azur, in May 2022, the Best Presentation Award at JEDNs (Days of the Doctoral School of Nice) Université Côte d'Azur, in June 2022, the Best Poster Award on Lipids, proteins and their interactions in organelle biology EMBO FEBS Lecture Course (Spetses – Greece) and in October 2022, the Best Poster Award at IPMC retreat.
In 2021, she went to Paris for a Cryo-EM Internship at Curie Institute in Dr Daniel Levy's Lab to learn how to reconstitute membrane proteins and there, she discovered Cyro-Microscopy. Her brillant article, recently published in Developmental Cell journal, is the result of the collaboration between both labs that she will present.
Bibiography:
- M Subra, M Dezi, J Bigay, S Lacas-Gervais, A Di Cicco, A Rita Dias Araújo, S Abélanet, L Fleuriot, D Debayle 1, R Gautier, A Patel, F Roussi, B Antonny, D Lévy, B Mesmin. VAP-A intrinsically disordered regions enable versatile tethering at membrane contact sites. Dev Cell 2023 Jan 23;58(2):121-138.e9. Doi: 10.1016/j.devcel.2022.12.010.
- Miserey-Lenkei S, Trajkovic K, D'Ambrosio JM, Patel AJ, Čopič A, Mathur P, Schauer K, Goud B, Albanèse V, Gautier R, Subra M, Kovacs D, Barelli H, Antonny B. A comprehensive library of fluorescent constructs of SARS-CoV-2 proteins and their initial characterisation in different cell types. Biol Cell. 2021 Jul;113(7):311-328. doi: 10.1111/boc.202000158. Epub 2021 May 10.
- Péresse T, Kovacs D, Subra M, Bigay J, Tsai MC, Polidori J, Gautier R, Desrat S, Fleuriot L, Debayle D, Litaudon M, Pham VC, Bignon J, Antonny B, Roussi F, Mesmin B. Molecular and cellular dissection of the oxysterol-binding protein cycle through a fluorescent inhibitor. J Biol Chem. 2020 Mar 27;295(13):4277-4288. doi: 10.1074/jbc.RA119.012012. Epub 2020 Feb 19. PMID: 32075908; PMCID: PMC7105299.
12:30
Dr Daniel LEVY, Research Director CNRS, Head of the Team Molecular Microscopy of Membranes

From : Institut Curie, UMR CNRS 168 Physical Chemistry Curie Unit, 26 rue d'Ulm PARIS
Email : daniel.levy@curie.fr
Team website : https://science.institut-curie.org/team-levy
Title of the lecture:
"3D architecture of membrane machineries by cryo-electron microscopy"
Abstract:
Today, cryo-electron microscopy (cryo- EM) is the most complete method to determine the three-dimensional (3D) structure of biological objects at resolutions that allow the construction of atomic models. All cellular components are concerned, whether they are purified (cytosolic and membrane proteins and membrane proteins, cytoskeleton filaments ribonucleic complexes, etc.) or associated with substrates or inhibitors, as well as the components present in eukaryotic cells, bacteria or viruses. Cryo-EM is also a multi-scale approach, which allows the characterization of biological samples from a few nanometers to a few microns, and to contextualize proteins within multi-protein functional assemblies, or in environments as complex as the interior of cells. I will introduce the principle of the method, especially cryo-tomography, and then describe some examples of 3D architecture of membrane proteins including proteins involved in membrane contact sites obtained in our team.
Biography:
Dr Daniel LEVY is a biophysicist interested to understanding major cellular functions in eukaryotic cells that involved membrane proteins of cell detoxification, intracellular traffic and communication between organelles and for which dysfunctions are involved in cancer, neurodegenerative diseases or viral infections.These functions are performed by machineries, heterogeneous in components, protein and membrane, membrane and filament as in 3D assemblies on membrane and involve protein and membrane interaction at short but also long distance. Dr Daniel LEVY designs in vitro reconstituted systems with a set of purified proteins and membrane models and describes the 3D architecture of proteins assemblies at subnanometric resolution by cryo-electron microscopy and cryo-electomography. Then in collaboration, he combines these results with data derived from cell biology and physics of membrane to get an integrated and mutltiscale view of the function.
Relevant Bibiography:
- M Subra, M Dezi, J Bigay, S Lacas-Gervais, A Di Cicco, A Rita Dias Araújo, S Abélanet, L Fleuriot, D Debayle 1, R Gautier, A Patel, F Roussi, B Antonny, D Lévy, B Mesmin. VAP-A intrinsically disordered regions enable versatile tethering at membrane contact sites. Dev Cell 2023 Jan 23;58(2):121-138.e9. Doi: 10.1016/j.devcel.2022.12.010.
- E de la Mora, M Dezi, A Di Cicco, J Bigay, R Gautier, J Manzi, J Polidori, D Castaño-Díez, B Mesmin, B Antonny, D Lévy. Nanoscale architecture of a VAP-A-OSBP tethering complex at membrane contact sites. Nat Commun 2021 Jun 8;12(1):3459. Doi: 10.1038/s41467-021-23799-1.
- RA Jani, A Di Cicco, T Keren-Kaplan, S Vale-Costa, D Hamaoui, I Hurbain, F-C Tsai, M Di Marco, A-S Macé, Y Zhu, M J Amorim, P Bassereau, J S Bonifacino, A Subtil, M S Marks, D Lévy, G Raposo, C Delevoye. PI4P and BLOC-1 remodel endosomal membranes into tubules. J Cell Biol. 2022 Nov 7;221(11):e202110132. Doi: 10.1083/jcb.202110132. Epub 2022 Sep 28.
- D Lévy, A Di Cicco, A Bertin, M Dezi. Cryo-electron microcopy for a new vision of the cell and its components (Article in French) Med Sci (Paris). 2021 Apr;37(4):379-385. Doi: 10.1051/medsci/2021034. Epub 2021 Apr 28.
- A Vial, C Taveneau, L Costa, B Chauvin, H Nasrallah, C Godefroy, P Dosset, H Isambert, K Xuan Ngo, S Mangenot, D Levy, A Bertin, P-E Milhiet. Correlative AFM and fluorescence imaging demonstrate nanoscale membrane remodeling and ring-like and tubular structure formation by septins. Nanoscale. 2021 Aug 7;13(29):12484-12493. Doi: 10.1039/d1nr01978c. Epub 2021 Jul 6.
Please feel free to contact academies.excellence4@univ-cotedazur.fr or mesmin@ipmc.cnrs.fr to make appointment for further discussion with Dr Daniel LEVY during his visit in NICE.
--------------------
ORGANIZERS:
Academy of Excellence 4 "Complexity & Diversity of the Living Systems"
PARTNERS
Academy of Excellence 5 "Human Societies, Ideas and Environments"
Graduate School and Research HEALTHY - Health Science Ecosystems
Graduate School and Research LIFE - Life and Health Sciences
Institute NeuroMod - Cognitive Systems, Normality and Pathology of the Human Brain and Computational Neurosciences
Labex SIGNALIFE - Network for Innovation on Signal Transduction Pathways in Life Sciences